In 1879, the German botanist Anton de Bary coined the term symbiosis. People usually think of symbiosis in terms of mutually beneficial relationships, but scientifically it’s a catch-all for any relationship—harmonious or antagonistic, parasitic or neighborly—between two different organisms. The details are left unclear.
Just two years later, while studying marine creatures under a microscope, de Bary’s compatriot Karl Andreas Heinrich Brandt realized that the small amber orbs lining their digestive tissues were not part of them, but a type of symbiotic algae. Brandt gave the cells the name “zooxanthellae,” which roughly means “little yellow cell in an animal.”
Rowan realized that DNA had the power to reveal what microscopes could not.
Among the creatures in whom zooxanthellae live are coral, and in the ensuing century scientists discovered that those little yellow cells donate a whopping 90 percent of the sugar they make from photosynthesis to their coral hosts. That energy is why coral colonies—which themselves are aggregations of pencil-eraser-sized polyps—can build reefs so immense they can be seen from outer space.
The break-up of the symbiosis, known as bleaching, which occurs when seawater warms, is the reason coral reefs are the first ecosystem-scale casualties of climate change. Half the world’s coral reefs have died, largely from bleaching, and by some predictions that number will rise to 99 percent by mid-century. Yet the nature of the relationship between coral and algae remains in many ways a mystery.
I fell in love with coral more than three decades ago. Back then no one imagined the dire conditions of today, so I didn’t start studying them because of any existential concern. Instead, I loved how math could explain the natural world; I wanted to study coral biomechanics, to understand the physical rules that limit the seemingly unfathomable diversity of coral forms and structures. Unfortunately, I had discovered this passion late in college and, with a single biology class on my transcript, was horribly underqualified for any graduate program.
Hoping to learn through osmosis, I wrangled a summer job at the venerated Marine Biological Laboratories in Woods Hole, Massachusetts. Along with scrubbing lab equipment, I fetched coffee and worked the photocopier in exchange for food, lodging, and being able to sit in on lectures with a marine ecology class of two dozen graduate students.
 A LACK OF COLOR: When stressed by heat, corals expel the photosynthetic algae living inside them. The algae provide corals with both energy and color; after they’ve departed the now-white corals are said to have bleached. Photo by Damsea / Shutterstock.
A LACK OF COLOR: When stressed by heat, corals expel the photosynthetic algae living inside them. The algae provide corals with both energy and color; after they’ve departed the now-white corals are said to have bleached. Photo by Damsea / Shutterstock.One of those students was Rob Rowan, tall and thin with blond tangled hair that made him look as if he’d either just woken up or emerged from the sea. Like me, Rowan was hoping to bolster his knowledge of marine science. He had trained as a classical geneticist, and I remember the awe I felt the first time he showed me a test tube of DNA he’d extracted.
Along with a German woman who studied crab larvae and a Californian surfer interested in ichthyology, the four of us formed a little symbiotic group, free diving every beach we could access and drinking gin and tonics late into the night talking about science. Thinking about the symbiosis between coral and algae today, a memory surfaces of Rowan saying late one night, “How’s it possible that all the coral, all 800 or so species, host just one species of zooxanthellae?”
Because zooxanthellae lack physical characteristics that distinguish them from one another, there was no visible way to tell if they were different species, even at high magnification. Rowan realized that their DNA had the power to reveal what microscopes could not.
After that summer in Massachusetts, Rowan went to Stanford. A few years later he and the late Dennis Powers published a genetic analysis of zooxanthellae in the journal Science.1 They “revolutionized the molecular phylogeny of Symbiodinium, as it was known at the time,” says Andrew Baker, a coral biologist at the University of Miami, calling zooxanthellae by their scientific name.
Maybe something about Durusdinium stresses coral out, toughening them up so they can withstand future conditions.
The work showed that zooxanthellae are not all the same. They discovered at least three species, preliminarily called A, B, and C. “But if you look closely at that paper,” Baker added, “there’s a species that does not have a label, which is from a Hawaiian coral. I think in all probability it’s a D—a Durusdinium.”
Durusdinium was left unnamed because it was historically rare, at the time probably making up less than 10 percent of coral symbionts. Much more numerous were the C’s, eventually called Cladocopium, which dominated throughout the Pacific and were found in equal abundance to the B’s, later called Breviolum, throughout the Caribbean. (The A’s held on to the name Symbiodinium.)
But Durusdinium was about to emerge from obscurity. In 1997-1998, a historic El Niño system heated waters and whitened coral along the coast of Panama and birthed the now familiar term: mass bleaching. “It became this big event that changed people’s perception of how important bleaching is to the fate of the reefs,” Baker says.
A few years earlier, Baker had arrived as a graduate student at the Smithsonian Tropical Research Institute in Panama, where Rob Rowan was doing postdoctoral research. They struck up a collaboration,2 and although Rowan had moved on when the historic heatwave struck, Baker was still in Panama. He was in the right place at the right time with the right scientific influences to study how corals changed when temperatures rose. “Not only did the corals that had the Durusdinium in them not bleach,” said Baker, “but those corals became more common.”
In a follow-up study3 of reefs in the Indian Ocean and the Red Sea, Baker and his colleagues found that, like in Panama, Durusdinium dominated where waters were warmest. The conclusion: Durusdinium were thermally tolerant.
During these years, Baker was captivated by a theory called the Adaptive Bleaching Hypothesis.4 “It was the idea that bleaching provides an opportunity for corals to dump existing symbionts,” explains Baker, “and then recover with different symbionts that in some cases are better-suited to the new environment and are therefore beneficial.” The finding that coral could shift to a thermotolerant symbiont following bleaching was called a “nugget of hope.”5
Relationships almost always involve trade-offs. In the early aughts, Australian scientists discovered that the nugget of hope was a bit tarnished. Experiments showed that juvenile coral hosting Durusdinium grew two to three times slower than their siblings hosting other symbionts. Subsequent work found that corals receive only about half as much sugar from Durusdinium as from other species of zooxanthellae.
Some researchers suggested that Durusdinium were, as one paper put it, “selfish opportunists” taking advantage of compromised coral to replace their more-beneficial competitors.6 Yet Baker believes that “people have been maybe too willing to label Durusdinium as being selfish. There are many competing ideas.”
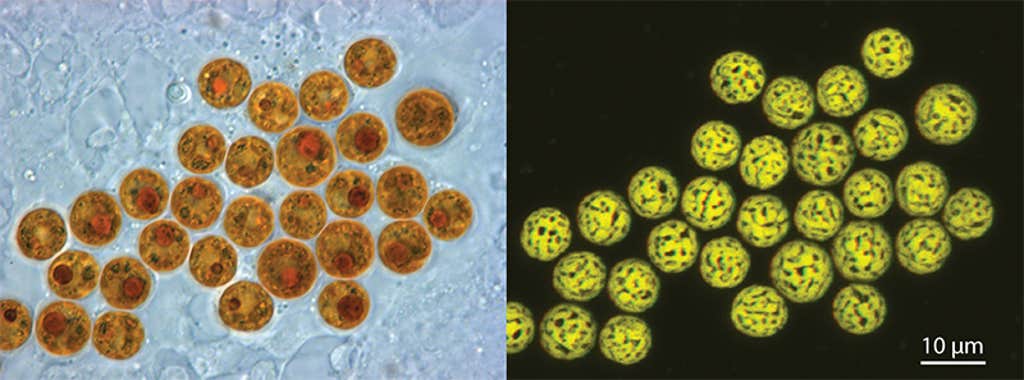 TINY PHOTOSYNTHESIZERS: Zooxanthella—the name translates roughly as “little yellow cell in an animal”—can be found inside corals and many other marine invertebrates. Those in coral were once thought to belong to a single genus. In fact there are several, and it’s hoped that especially heat-tolerant strains will allow corals to survive as oceans warm. Image by Allison M Lewis / Wikimedia Commons.
TINY PHOTOSYNTHESIZERS: Zooxanthella—the name translates roughly as “little yellow cell in an animal”—can be found inside corals and many other marine invertebrates. Those in coral were once thought to belong to a single genus. In fact there are several, and it’s hoped that especially heat-tolerant strains will allow corals to survive as oceans warm. Image by Allison M Lewis / Wikimedia Commons.One hypothesis suggests that because Durusdinium abundance is comparatively low—a coral hosting them may contain several hundred, whereas thousands of Breviolum or Cladocopium dwell within each of their coral hosts—they give the impression of selfishness, but on a per-cell basis supply just as much sugar. “It could be that Durusdinium are doing the best they can, you know?” Baker says.
Yet another hypothesis posits that something about Durusdinium stresses coral out, toughening them up so they can withstand future conditions, much as army recruits are stressed to prepare them for combat. Which, one could argue, isn’t really selfish. Then there’s the so-called Tenacious D hypothesis, which proposes that Durusdinium isn’t necessarily heat-tolerant. Rather, they evade notice by coral immune systems, which play a role in the bleaching process and may become more reactive during periods of heat-induced stress.
If Durusdinium were selfish, they would act like parasites, feeding their host just barely enough to keep it alive. There’s some evidence for this: Baker and his colleagues followed the fates of more than 100 corals around the central Pacific island of Kiribati during a severe, 10-month-long heat wave.7 As expected, corals already hosting Durusdinium didn’t bleach—but few survived. Perhaps they were too weakened by Durusdinium’s sparse rations to withstand the stress. However, the study also followed colonies that hosted Cladocopium before the heatwave. As expected, they bleached, but then shifted to Durusdinium and more of these colonies survived. Selfishness is a complicated thing; it depends a lot on context.
Perhaps bleaching isn’t just about the coral, but about the algae fleeing a bad situation.
While Rob Rowan was deciphering zooxanthellae genomes, I finally cobbled together a resume strong enough to be admitted to graduate school in California. The professor who accepted me into his lab studied echinoderm biomechanics. While they weren’t coral, I was transfixed by the crystalline spaceship forms of sea urchin larvae and hoped I would someday take those biomechanical insights to the world of coral.
A few months in though, I began to wither. My advisor was distant and gruff; my impostor syndrome intensified. Then, my advisor announced that he was moving to another university in Oregon. I could apply to transfer and remain in his lab, or be released into the pool of academia, free to search for a new opportunity—if I could find one.
When a coral bleaches, we see the white skeleton that remains, like a specter of the reef that once was. But what of the other partner, the golden orbs? The algae regrow their flagellae and swim away. Out in the sea, algae must contend with predation and gathering nutrients, but many survive. That’s yet another hypothesis: Perhaps bleaching isn’t just about the coral, but about the algae fleeing a bad situation.
I did find my way into another lab, though not to study coral or biomechanics. My dissertation focused on turning satellite images of the ocean into maps of phytoplankton photosynthesis, the same process that zooxanthellae perform inside of coral, and which protects us from climate change by removing carbon dioxide from Earth’s atmosphere. It was the best I could do and while the work sustained me for a bit, eventually I faded from scientific research.
In 2014, near Miami, coral researchers noticed that many brain coral, maze coral, and boulder coral were dying, their tissue melting away over the course of just a few weeks. The devastating disorder became known as Stony Coral Tissue Loss Disease. Today, the disease has spread from the Florida Keys and become epidemic throughout the Caribbean. Its pathogen, and therefore any cure, remains unknown.
Most of the corals that fall prey to Stony Coral Tissue Loss Disease host the species of zooxanthellae called Breviolum. One of Andrew Baker’s graduate students, Caroline Dennison, performed experiments bleaching Breviolum from the coral and then providing them with Durusdinium. When she introduced Stony Coral Tissue Loss Disease, the corals hosting Durusdinium were two to three times less susceptible to the disease.8
“Here’s a good example of a totally unexpected benefit of hosting Durusdinium,” said Baker. “Some wacky new disease crops up and it turns out that if you’ve got Durusdinium, you’re not as susceptible.”
I frequently wonder what would have happened if the dream I had back at Woods Hole had come true. What if I had been admitted to a lab to study coral biomechanics? Would I have had a long career doing what I love? Or would my relationship with academics have soured under the constant grind of writing grants and teaching? Would I have resented the tenure track, being forced to live where a job was available, living life semester by semester?
It’s easy with hindsight to feel that bleaching out of academics led me to writing, which unexpectedly led me back to coral in my most recent book, on my own terms. Maybe it’s even more important now, with coral so threatened, that my writing reaches more people than any biomechanical equations I might have worked out. Perhaps I experienced my own adaptive bleaching.
Back in 2010, I reached out to Rob Rowan for a story I was writing about coral. He was working at the University of Guam and said that the grind of academics was wearing on him. He wanted to retire early. Baker heard that Rowan had left Guam, but I could find no announcement. Neither of us know how to contact Rowan anymore. He doesn’t turn up in internet searches. Rowan, who inspired both me and Baker, who discovered so much about the ocean’s most important symbiosis, has drifted away without a trace. Sometimes relationships don’t survive.
That may ultimately be the case for coral and algae as well. But right now, below the waves, Durusdinium are swimming wherever coral live. As temperatures rise, more of these golden orbs are finding their way into symbiosis with coral. Will these new relationships be harmonious or antagonistic, tenacious or selfish, protective or debilitating, or something else entirely?
Scientists like Andrew Baker are still trying to figure it out. “I hesitate to say it will save them,” he said. “But it’s definitely going to be a big part of their biology.” ![]()
Lead image: Damsea / Shutterstock
References
1. Rowan, R. & Powers, D.A. A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbiosis. Science 251, 1348-1351 (1991).
2. Rowan, R., Knowlton, N., Baker, A., & Jara, J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265-269 (1997).
3. Baker, A.C., Starger, C.J., McClanahan, T.R., & Glynn, P. W. Corals’ adaptive response to climate change. Nature 430, 741 (2004).
4. Buddemeier, R.W., Baker, A.C., Fautin, D.G., & Jacobs, J.R. The adaptive hypothesis of bleaching. In Rosenberg, E. & Loya, Y. (eds.) Coral Health and Disease Springer, New York, NY (2004).
5. Berkelmans, R. & van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B 273, 2305-2312 (2006).
6. Stat, M. & Gates, R.D. Clade R Symbiodinium in Scleractinian corals: A “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? Journal of Marine Science 2011 (2010).
7. Claar, D.C., et al. Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nature Communications 11, 6097 (2020).
8. Dennison, C.E., et al. The role of algal symbionts (genus Breviolum) in the susceptibility of corals to Stony Coral Tissue Loss Disease in South Florida. Florida Department of Environmental Protection (2021).


